Urogenital Mycobacterium Tuberculosis: What Should Urologists Know?
1. Executive Summary
-
- M. tuberculosis is a bacterium that causes tuberculosis (TB).
- Globally, there were 10.4 million new cases of TB in 2016, with 1.7 million deaths.
- In the same year in the U.S., there were 9,272 new cases of TB, with no reported deaths from TB that year.
- Although the incidence of TB is declining, extrapulmonary TB is increasing as a percentage of TB cases.
– While there is a significant decline in the incidence of pulmonary TB, extra-pulmonary TB has seen only a slight decline.
– The genitourinary tract is a primary target of hematogenous infections and is the most common site of extra-pulmonary TB.
-
- Genitourinary tract TB infections have also been reported to occur through sexual transmission.
- Inasmuch as drug resistance is a major world-wide problem, identification of resistance is necessary prior to commencing treatment.
– Genotyping is the primary method for identifying resistance in M. tuberculosis.
- Treatment of TB typically involves using a combination of drugs.
II. Introduction
Tuberculosis (TB) is the most common worldwide cause of mortality from infectious diseases, with nine million new cases and two million deaths per year. The World Health Organization (WHO) reported in 2014 that approximately one-third of the world’s population has latent tuberculosis.1 Developing countries suffer the greatest burden from TB, having about 95% of all cases.2
The resurgence of TB has been noted in both endemic and non-endemic regions, mainly due to increased migration, the human immunodeficiency virus (HIV) pandemic, and the emergence of drug-resistant strains of Mycobacterium tuberculosis (MTB).2 Multi-drug resistant tuberculosis (MDR-TB), caused by strains of Mycobacterium tuberculosis complex that are resistant to two of the most potent front-line tuberculosis drugs, isoniazid and rifampin, presents a substantial obstacle to treatment of TB.3 Extensively drug-resistant TB (XDR-TB) is a rare type of MDR-TB: in addition to resistance to isoniazid and rifampin, it is also resistant to any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).4
WHO estimates that in 2015, 3.9% of previously untreated and 21% of previously treated TB cases worldwide had MDR-TB. The annual worldwide incidence of MDR-TB is 580,000, with about 250,000 deaths attributable to MDR-TB. About 10% of MDR-TB patients are XDR-TB.5
Greater drug resistance leads to increased cost for treatment. Direct costs (drugs and diagnostics, case management social work, housing and transportation, and hospitalization) range from $18,000 to treat drug-susceptible TB to $513,00 to treat extremely resistant disease.
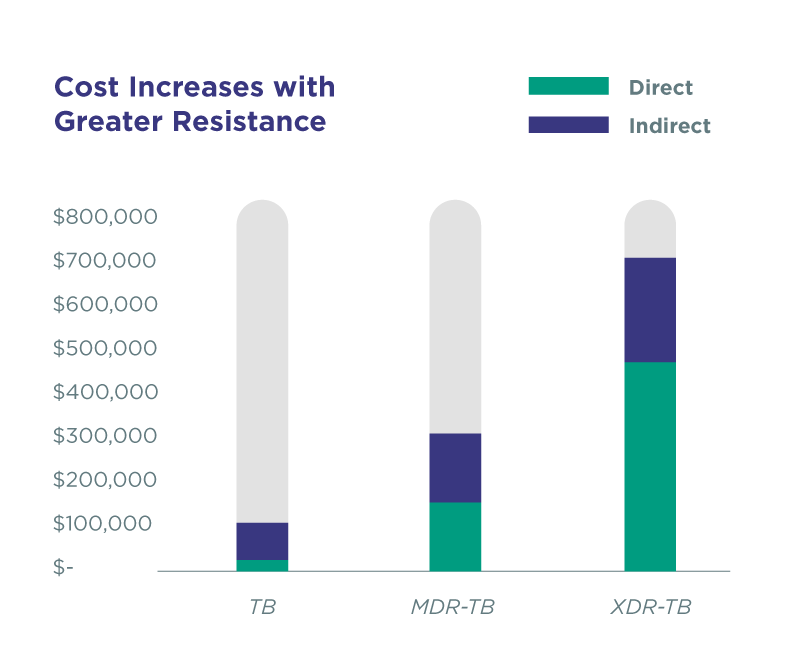
A relative increase in extra-pulmonary TB has been reported due to a significant decline in pulmonary tuberculosis (PTB) and an only modest decline in extra-pulmonary TB. The genitourinary tract is a primary target of hematogenous infections and is the most common site of extra-pulmonary TB.2
Urogenital TB (UGTB) is the second most common type of TB in areas where TB is epidemic, and the third most common form in regions with a low incidence of TB.1 UGTB may occur through hematogenous spread from pulmonary TB. Alternatively, generalized TB may occur initially as UGTB. Further, UGTB is often either missed (because UGTB has no unique and specific symptoms) or misdiagnosed (because it can be masked by other diseases, such as UTI, cancer, or urolithiasis).7 The prevalence of UGTB among UTI patients with poor results from antibiotic treatment has been reported as 25.8%, with a comorbidity of UTI and UGTB at 65.1%.8
TB of the prostate can often be overlooked: 77% of all men that died from all forms of TB had prostate TB. Further, prostate TB may cause TB to be spread as a sexually transmitted disease.9
III. Clinical relevance
UGTB usually affects adults between the second and fourth decades of life (mean age = 40.7 years) and is reported as being rare in children and in the fifth and sixth decades.2
Tuberculosis can be a difficult disease to diagnose, with myriad presentations and manifestations.10 The insidious onset and non-specific constitutional symptoms of genitourinary tuberculosis (UGTB) often lead to delayed diagnosis and rapid progression to a non-functioning kidney. Due to hematogenous dissemination of TB, there is a potential risk of involvement of the contralateral kidney.2 TB of the ureter most commonly develops in the lower third of the ureter, but multiple lesions are also possible. Incorrect therapy may lead to the development of ureteral stricture that may result in the loss of kidney, even if TB is finally cured.9
Complications of UGTB are well-documented, including sinus tracts, fistulae, amyloidosis, and tuberculous interstitial nephritis (TIN). TIN can be difficult to diagnose, with delayed or poor treatment leading to renal failure.2
Worldwide, 15% of TB patients are co-infected with HIV, and in HIV-endemic areas, as many as 75% of patients with UGTB are co-infected with HIV.2
Prostate TB is an important issue in men. It is often under-diagnosed and poorly treated. Prostate TB may lead to sexual transmission of TB, as well as infertility and chronic pelvic pain.9 Among patients with pulmonary TB, 28% also had prostate TB, and 77% of men who died from tuberculosis of all localizations had prostate tuberculosis, also.7
Extensive worldwide use of anti-TB drugs has enabled resistant TB bacteria to proliferate and acquire more mutations, and for MDR strains to emerge and spread. It was thought that resistant strains were less virulent and transmissible than susceptible strains, leading to greater emphasis on poor treatment compliance as the main source of drug-resistant TB. Treatment for TB is a lengthy process, and strict adherence to the regimen, especially in low-income settings, can be difficult.
However, the perspective on emergence of drug-resistant TB is changing. Molecular epidemiology tools have enabled researchers to show that primary resistance—the transmission of drug-resistant TB from one person to another—is playing a much larger role in the spread of drug-resistant TB than previously thought.11 This perspective is supported by a recent study, published in The Lancet Infectious Diseases in December, genotyping of isolates from TB patients in Shanghai, China, showed that person-to-person transmission accounted for 73% of MDR-TB cases overall.12
IV. Value of genotyping and phenotyping
Multidrug-resistant (MDR) tuberculosis, defined as disease caused by M. tuberculosis that is resistant to both rifampin and isoniazid (and frequently other drugs) presents numerous complications. It has emerged as a global threat and occurs more frequently in previously treated cases (secondary resistance) than in untreated patients (primary resistance).13 Because the resistant strains were then transmitted to treatment-naive patients, drug resistance testing for M. tuberculosis was recommended for all definite cases of tuberculosis.14 However, drug resistance testing was estimated to be performed in only 58% of previously treated TB cases (12% of untreated cases).13
Treatment of MDR-TB is long, expensive and toxic; errors in the design of the regimen are associated with increased rates of failure and death.15
“Whenever possible, the initial treatment regimen should be individually tailored according to the results of drug-susceptibility testing of the M. tuberculosis isolate from the patient, with testing performed either by culture or with the use of DNA-based methods. In the absence of this information, empirical regimens can be used, but as soon as the results of drug-susceptibility testing become available, the treatment regimen should be adjusted.”16
Drug-resistant tuberculosis regimens need to include a sufficient number of effective drugs, a significant challenge for clinicians worldwide, as most are forced to make therapy decisions without any drug susceptibility testing (DST) information.15 WHO policy guidance for the use of novel antituberculosis drugs (bedaquiline and delamanid) and newly developed shorter regimens for the treatment of drug-resistant TB requires rapid diagnosis and triaging of patients to identify those who are most likely to benefit from the new treatment options.5
Approximately 20% of worldwide TB cases are resistant to at least one of the first- or second-line anti-TB drugs; 5% are resistant to both isoniazid and rifampin, the most potent and commonly used first-line antibiotics. About 580,000 new cases of MDR-TB occur each year, with about 10% of those being either XDR-TB or totally drug resistant.11
Diagnosis of TB has changed significantly in the last two decades. Culture has been the standard for both diagnosis and drug-susceptibility testing, but molecular DNA–based diagnostics have become widely available and permit both rapid diagnosis and preliminary assessment of drug susceptibility. Further, this approach enables prompt initiation of treatment regimens that can be tailored to individual patients.16
A total of 286 TB genetic alterations associated with phenotypic resistance (111 mutations plus 150 frameshifts and 25 premature stop codons) have been identified.15 Resistance can often be predicted by identifying resistance-related mutations using genotyping. A number of papers have examined correlations between genotyping and phenotyping in predicting antibiotic resistance in the context of TB. One such study assessed genotyping to predict resistance against phenotypic expression of resistance in 250 patients in Hong Kong.17 Table 1 shows their results.
Table 1. Summary and correlation of genetic and phenotypic characteristics of 250 clinical MTB isolates
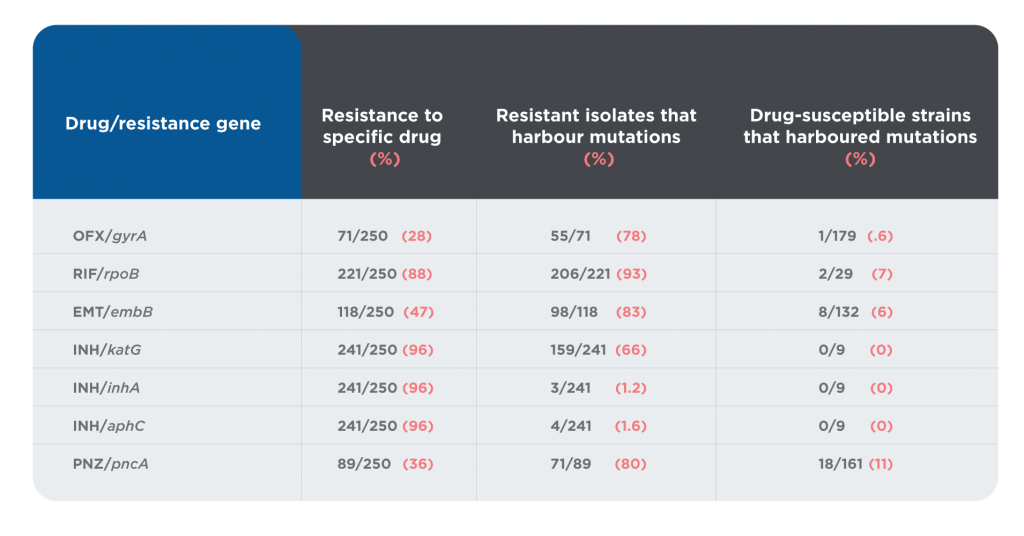
The overall statistics for the study showed that 69.8% of patient samples displayed resistance of some kind. A resistant genotype was identified in 48.8% of samples exhibiting resistance. However, the distribution was uneven: 93% of samples with the RIF/rpoB genotype showed resistance, while only 1.2% of samples with the INH/inha genotype showed resistance. Of those samples that were drug susceptible, 5.5% contained resistance mutations.
There are several possible explanations for differences between resistance testing by genotype and by phenotype:
-
- It is likely that not all resistance mutations have been identified, and mutations continue to occur.
-
- Point mutations within a gene conferring resistance can significantly modify the level of resistance.18
-
- Different resistance mutations lead to distinct minimum inhibitory concentrations, some of which may still be overcome by increased dosing.19
-
- The ‘critical concentration’ varies with the medium used (egg-based media, such as Loewensteine-Jensen, which are cooked at 80C, and synthetic Middlebrook (7H9, 7H10, 7H11 or 7H12) media to which oleic acid albumin, dextrose and catalase are added) because this value depends on the concentration of the drug that remains active in the medium.13
</ul
It is clear that both genotyping and phenotyping provide valuable evidence concerning drug susceptibility, but that neither provides the complete picture. Both are necessary, inasmuch as neither is sufficient alone.
V. Diagnostic algorithm
One of the most pressing issues in managing TB is diagnosis: 40% of all new cases are not reported. These cases are either undiagnosed or diagnosed but not reported.20
Clinical features of UGTB have no specific signs, are unstable and depend on many factors; this is one of the reasons for late diagnosis. Diagnosis depends on performance of a number of components, including a medical history, physical examination, chest radiograph, diagnostic microbiology, and drug resistance.
Although many agencies still call for testing for TB infection, usually using the Mantoux tuberculin skin test21, this may be of little usefulness. The Mantoux test is positive in >90% of TB patients, but it has no value in regions with a severe epidemic situation, such as China, Russia, India, and Pakistan, where almost all adults are infected with TB and thus all have a positive skin tuberculin test.1
A negative chest radiograph and tuberculin test cannot exclude the diagnosis of extra-pulmonary TB. Only 36.5% of patients with UGTB have a previous diagnosis of TB, or abnormal imaging studies. Evidence of active TB or an abnormal chest radiograph is present in less than 50% of cases. Only 20-30% of UGTB patients will have a previous history of PTB; an additional 25-50% will have radiographic evidence of prior subclinical PTB.2
The diagnosis of urogenital TB is confirmed if Mycobacterium tuberculosis (MTB) is detected, but in recent years, MTB could be found in only half of TB patients. Therefore, in patients suspected of having urogenital TB, but without documented evidence of MTB, the diagnosis of urogenital TB has to be made on the basis of other features, such as a skin test, histologic findings, caverns revealed by intravenous pyelography, or sterile pyuria.1
The sensitivity and specificity of detecting and identifying TB could be improved through use of PCR. In a large meta-analysis, PCR used to detect pulmonary TB was found to have a sensitivity of 82% and a specificity of 99%. The same study reported that PCR used to detect extrapulmonary TB had a sensitivity of 70% and a specificity of 99%.22
Positive identification of MTB confirms a diagnosis of TB. Resistance testing, as discussed in the section above, is necessary to guide treatment.
VI. Treatment algorithm
“Multidrug-resistant (MDR) tuberculosis, defined as disease caused by M. tuberculosis that is resistant to both rifampin and isoniazid (and frequently other drugs), is complicated, and treatment should always be guided by an experienced physician. Whenever possible, the initial treatment regimen should be individually tailored according to the results of drug-susceptibility testing of the M. tuberculosis isolate from the patient, with testing performed either by culture or with the use of DNA-based methods. In the absence of this information, empirical regimens can be used, but as soon as the results of drug-susceptibility testing become available, the treatment regimen should be adjusted.”16
While TB is curable when patients adhere to the treatment regimen, MDR- and XDR-TB are more problematic. Treatment options are limited, expensive, and often toxic, and drug therapy can last up to two years. Mortality rates of about 40% have been reported for MDR-TB, and 60% for XDR-TB. And while China, India, Russia, and South Africa have the highest burden of MDR- and XDR-TB, widespread international travel and migration means drug-resistant TB has no borders.11
The standard treatment regimen for presumably drug-susceptible tuberculosis includes an induction phase consisting of rifampin, isoniazid, and pyrazinamide, to which ethambutol is added as protection against unrecognized resistance to one of the three core drugs. Once susceptibility to isoniazid, rifampin, and pyrazinamide has been confirmed, ethambutol can be discontinued. In young children, this drug is frequently omitted if the source of transmission is known to have drug-susceptible tuberculosis, because recognizing the toxic effects of ethambutol is challenging in children. The induction phase is followed by a consolidation phase consisting of rifampin and isoniazid for an additional 4 months of treatment.16
In the case of resistance to isoniazid (or unacceptable toxic effects associated with isoniazid) in the absence of rifampin resistance, a standard 6-month regimen in which isoniazid is replaced by a later-generation fluoroquinolone (levofloxacin or moxifloxacin) is likely to lead to a similar treatment outcome, and a 6-month regimen containing rifampin, moxifloxacin, pyrazinamide, and ethambutol for 2 months, followed by rifapentine and moxifloxacin for 4 months, was recently shown to be effective.16
When the disease is naive and caused by drug-sensitive MTB, first-line anti-TB drugs should be prescribed. When there is resistance of MTB to first-line anti-TB drugs or poor tolerance, severe adverse effects, and recurrence of the disease, second- or third-line anti-TB drugs are indicated.1
Table 2. Classification of antituberculosis drugs1
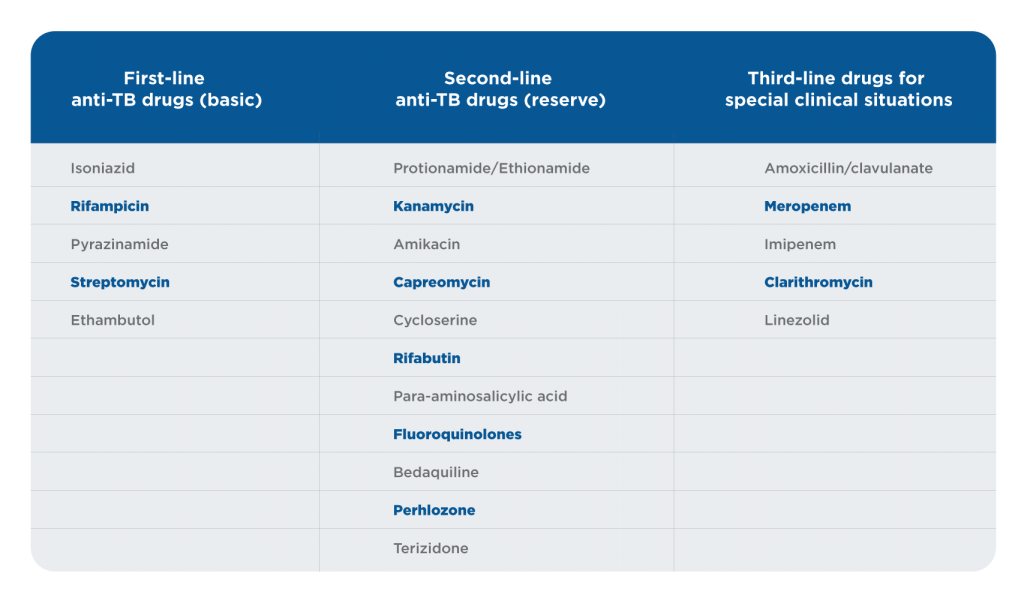
The treatment of urogenital TB differs from the treatment of pulmonary TB. Streptomycin and kanamycin are not recommended for urogenital TB. Ofloxacin and levofloxacin are the only fluoroquinolones suitable to treat urogenital TB. Moxifloxacin and sparfloxacin are respiratory fluoroquinolones that are suitable for pulmonary TB but not optimal for urogenital TB. PAS is recommended for urogenital TB with involvement of the pelvic organs because it provides an antiprostaglandin, anti-inflammatory effect. Amoxicillin/clavulanate should be prescribed together with meropenem or imipenem because they potentiate the anti-TB effect. Cycloserine is recommended if the patient has significant comorbidity and a nonspecific UTI [16]. Patients with urogenital TB and HIV who are receiving antiretroviral therapy should be treated with rifabutin instead of rifampicin. Rifampicin and streptomycin are contraindicated in patients after organ transplantation. Amikacin, streptomycin, and kanamycin are contraindicated for urinary tract TB patients because these drugs provoke a transformation from TB inflammation to fibrosis.1
Five regimens of chemotherapy are used depending on the form of urogenital TB. Table 3 presents the standard regimens of anti-TB treatment. Regimen 1 is applied in newly diagnosed treatment-naive patients with drug-susceptible (or if there was no growth of MTB) uncomplicated kidney TB stages 1 and 2, isolated TB epididymitis, patients who were diagnosed by histology after organ removal, and if there is no other TB focus. Regimen 2 is applied in newly diagnosed treatment-naive patients with drug-susceptible (or if there was no growth of MTB) uncomplicated kidney TB stages 3 and 4. Regimen 3 is applied in newly diagnosed treatment-naive patients with drug-susceptible (or if there was no growth of MTB) kidney TB stage 4, kidney TB of any stage complicated by urinary tract TB, and prostate TB. Regimen 4 is applied in patients with relapse of urogenital TB, with high risk of multidrug resistance (MDR), independent of form and stage. Regimen 5 is applied in patients with MDR urogenital TB, independent of form and stage. Every regimen starts with a phase of intensive therapy (2–4 mo) followed by the continuation phase (Table 3).1
Table 3. Standard regimens of chemotherapy for urogenital tuberculosis1
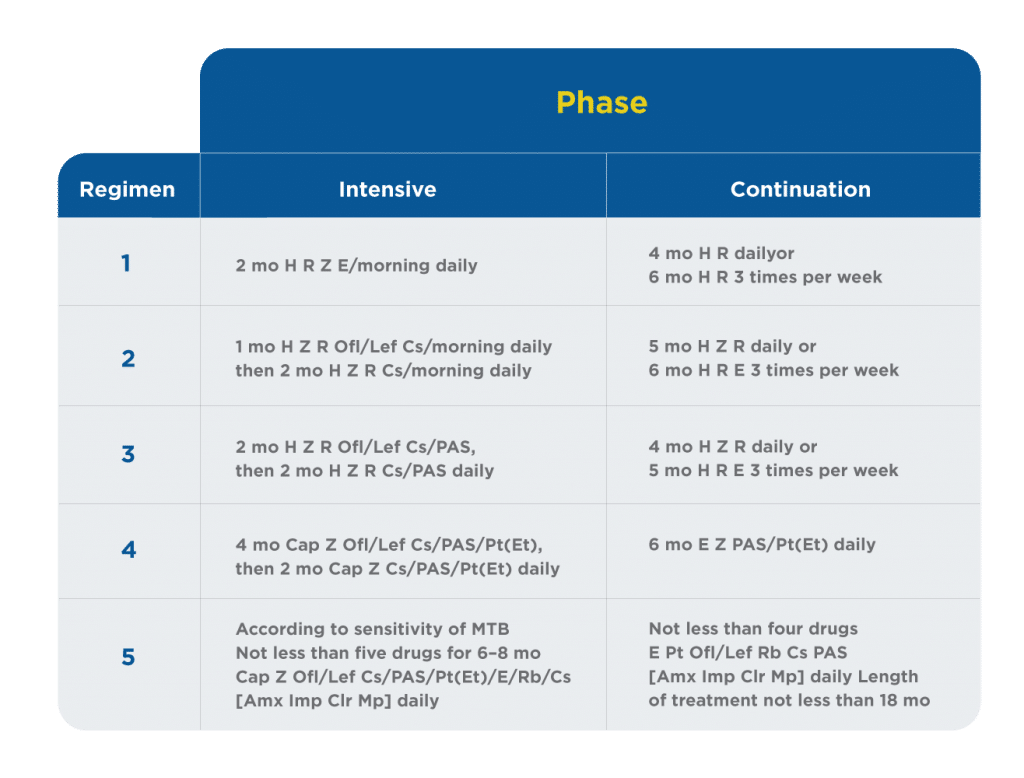
– Amx = amoxicillin/clavulanate;
– Cap = capreomycin;
– Clr = clarithromycin;
– Cs = cycloserine;
– E = ethambutol;
– H = isoniazid;
– Imp = imipenem;
– Mp = meropenem;
– Ofl/Lef = ofloxacin/levofloxacin;
– PAS = para-aminosalicylic acid;
– Pt(Et) = protionamide (ethionamide);
– R = rifampicin;
– Rb = rifabutin;
– Z = pyrazinamide.
Supplemental therapy is prescribed to minimize the negative consequences of anti-TB chemotherapy, to prevent excessive development of fibrosis, to decrease toxicity, to save the function of an organ, and to improve healing. Examples of medications for the supplemental treatment of urogenital TB are tocopherol, canephron, and trospium chloride for bladder TB stage 3 and afala and prostanorm for prostate TB.1
VII. Summary
UGTB is a frequent form of TB, but it is a mostly overlooked disease. Despite major efforts to increase case detection, an estimated one-third of new TB cases are still being missed each year.10 UGTB presents unique challenges to appropriate diagnosis, treatment, and management. Detection by PCR and determination of resistance through genotyping and phenotyping facilitate optimal treatment of UGTB.
References
1. Kulchavenya, E., Naber, K. & Bjerklund Johansen, T. E. Urogenital Tuberculosis: Classification, Diagnosis, and Treatment. European Urology Supplements 15, 112–121 (2016).
2. Merchant, S., Bharati, A. & Merchant, N. Tuberculosis of the genitourinary system-Urinary tract tuberculosis: Renal tuberculosis-Part I. Indian J Radiol Imaging 23, 46–63 (2013).
3. Berrada, Z. L. et al. Rifabutin and Rifampin Resistance Levels and Associated rpoB Mutations in Clinical Isolates of Mycobacterium tuberculosis Complex. Diagn Microbiol Infect Dis 85, 177–181 (2016).
4. Fact Sheets | Drug-Resistant TB | Extensively Drug-Resistant Tuberculosis (XDR TB) | TB | CDC. (2018). Available at: https://www.cdc.gov/tb/publications/factsheets/drtb/xdrtb.htm. (Accessed: 29th July 2018)
5. Falzon, D. et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 49, (2017).
6. THE COSTLY BURDEN OF DRUG-RESISTANT TB IN THE U.S. 1
7. Kulchavenya, E. & Kholtobin, D. Diseases masking and delaying the diagnosis of urogenital tuberculosis. Ther Adv Urol 7, 331–338 (2015).
8. Kulchavenya, E. & Cherednichenko, A. Urogenital tuberculosis, the cause of ineffective antibacterial therapy for urinary tract infections. Ther Adv Urol 10, 95–101 (2017).
9. Kulchavenya, E. Urogenital tuberculosis: definition and classification. Ther Adv Infect Dis 2, 117–122 (2014).
10. Kulchavenya, E. & Dubrovina, S. Typical and unusual cases of female genital tuberculosis. IDCases 1, 92–94 (2014).
11. Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. The Lancet Respiratory Medicine 5, 291–360 (2017).
12. Yang, C. et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. The Lancet Infectious Diseases 17, 275–284 (2017).
13. Schön, T. et al. Mycobacterium tuberculosis drug-resistance testing: challenges, recent developments and perspectives. Clinical Microbiology and Infection 23, 154–160 (2017).
14. Heifets, L. B. & Cangelosi, G. A. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int. J. Tuberc. Lung Dis. 3, 564–581 (1999).
15. Miotto, P. et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J 50, (2017).
16. Horsburgh, C. R., Barry, C. E. & Lange, C. Treatment of Tuberculosis. New England Journal of Medicine 373, 2149–2160 (2015).
17. Chan, R. C. Y. et al. Genetic and phenotypic characterization of drug-resistant Mycobacterium tuberculosis isolates in Hong Kong. J Antimicrob Chemother 59, 866–873 (2007).
18. Jamieson, F. B. et al. Profiling of rpoB Mutations and MICs for Rifampin and Rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 52, 2157–2162 (2014).
19. Ruesen, C. et al. Linking minimum inhibitory concentrations to whole genome sequence-predicted drug resistance in Mycobacterium tuberculosis strains from Romania. Sci Rep 8, (2018).
20. Chin, D. P. & Hanson, C. L. Finding the Missing Tuberculosis Patients. J Infect Dis 216, S675–S678 (2017).
21. Fact Sheets | Testing and Diagnosis | Fact Sheets – Diagnosis of Tuberculosis Disease | TB | CDC. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/diagnosis.htm. (Accessed: 29th July 2018)
22. Babafemi, E. O., Cherian, B. P., Banting, L., Mills, G. A. & Ngianga, K. Effectiveness of real-time polymerase chain reaction assay for the detection of Mycobacterium tuberculosis in pathological samples: a systematic review and meta-analysis. Syst Rev 6, (2017).